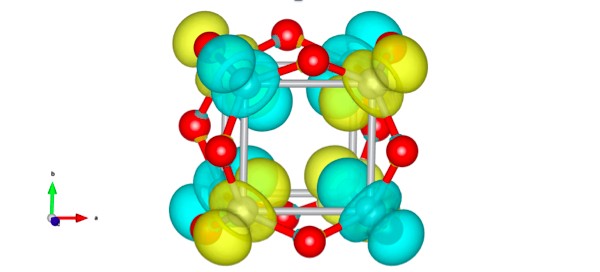
The researchers have found the unexpectedly stable titanium oxide cluster Ti8O12 by searching through all of Ti8On (n = 1-18) clusters. At the origin of the particular stability of bare Ti8O12 cluster is unique chemical bonding where eight electrons of Ti atoms interact with each other in antiferromagnetic fashion to lower the total energy of the system. Artem Oganov, professor at Skoltech, gives a comment:
“We have studied titanium oxide nanoparticles and discovered an “island of stability” – the bare Ti8O12 cluster. Ti8O12 is rather different from other clusters despite its high geometric symmetry. This stable particle is antiferromagnetic: there are opposite magnetic moments at neighboring Ti atoms that compensate each other. This type of magnetic ordering is common in crystals, but was not discovered in nanoparticles before.”
Authors – Yu (MIPT), Qian (Stony Brook University), Popov and Boldyrev (Utah State University), – suppose that the results of the research will inspire their colleagues. There is a possibility that the Ti8O12 particle can be made in the lab and one day we will be able to see it using micro- (or nano-?) scope.
Evolutionary algorithm USPEX and DFT+U calculations showed that the complex has a highly symmetric geometric structure: 8 titanium atoms form a cube that contains the O atoms near midpoints of its edges.
Titanium oxides excite great interest because of their photoactivity and their widespread industrial use: for example, titanium oxychloride is of interest in the semiconductor industry or for water and air filtration systems. The Ti8O12H8 cluster can be used as an ideal model to study the catalytic transformation of methanol to formaldehyde.
Here is the question: how does the bare Ti8O12 cluster manage to maintain stability? Previously, the authors have detected unusual chemical bond patterns in the clusters of transition metal oxides. But the observation of electrons, which interact with each other in an antiferromagnetic fashion to lower the total energy of the system, exceeded expectations.
Scientists found twelve stable clusters: Ti8, Ti8O1, Ti8O2, Ti8O3, Ti8O5, Ti8O8, Ti8O10, Ti8O12, Ti8O13, Ti8O14, Ti8O16 and Ti8O17. During the analysis of their stability, the basic premises of thermodynamics (the calculation of the free energy of Gibbs and Helmholtz free energy) were used. The result is: Ti8O12 has both a wide stability field and high symmetry.
The research group plans to continue research in this area and also to expand the predictive power of their USPEX method.
Link: http://dx.doi.org/10.1002/anie.201508439
*****
The Skolkovo Institute of Science and Technology (Skoltech) is a private graduate research university in Skolkovo, Russia, a suburb of Moscow. Established in 2011 in collaboration with MIT, Skoltech educates global leaders in innovation, advances scientific knowledge, and fosters new technologies to address critical issues facing Russia and the world. Applying international research and educational models, the university integrates the best Russian scientific traditions with twenty-first century entrepreneurship and innovation.
Contact information:
Skoltech Communications
+7 (495) 280 14 81