Scientists from Skoltech, Institute for Problems of Chemical Physics of RAS and MSU have studied a partial replacement of lead in the active layer of the perovskite solar cells with uni-, bi-, tri- and tetravalent metal ions. They have revealed the influence of such lead substitution on the optoelectronic properties of the perovskite material and photovoltaic performance of the devices. The obtained results allowed researchers to propose a model for predicting successful replacements for lead in order to decrease the toxicity of the perovskite absorbers while maintaining their advanced optoelectronic properties essential for efficient operation of solar cells. This work also provided some understanding of the significant deterioration of the perovskite solar cell performance when lead is partially or completely replaced with less toxic tin or germanium.
 Design of hybrid perovskite solar cells based on complex lead halides APbI3 (A –univalent organic cation) represents one of the most rapidly developing fields of scientific research for the last 2-3 years. Perovskite solar cells have demonstrated impressive solar light power conversion efficiency of >22%, thus coming very close to the most advanced crystalline silicon devices. However, hybrid perovskite solar cells can be potentially much cheaper compared to the silicon or thin-film chalcogenide photovoltaics, since they can be produced using solution-based low-cost high-throughput roll-to-roll processes. This allows one to consider perovskite solar cells as the most promising emerging photovoltaic technology.
Design of hybrid perovskite solar cells based on complex lead halides APbI3 (A –univalent organic cation) represents one of the most rapidly developing fields of scientific research for the last 2-3 years. Perovskite solar cells have demonstrated impressive solar light power conversion efficiency of >22%, thus coming very close to the most advanced crystalline silicon devices. However, hybrid perovskite solar cells can be potentially much cheaper compared to the silicon or thin-film chalcogenide photovoltaics, since they can be produced using solution-based low-cost high-throughput roll-to-roll processes. This allows one to consider perovskite solar cells as the most promising emerging photovoltaic technology.
Unfortunately, practical implementation of perovskite solar cells is hampered by their low operation stability and relatively high toxicity of the used materials. Therefore, there is a great interest to the development of some alternative less toxic perovskite materials with improved properties. One the approaches pursued by many research laboratories is based on a partial or complete replacement of certain ions in the conventional APbI3 perovskite structure. Special attention is paid to tin and germanium as environment-friendly alternatives to lead. However, solar cells based on perovskite materials incorporating tin or germanium show very low efficiencies despite the efforts of many research groups worldwide.
A significant progress has been achieved in the recent work carried out by a joint team of researchers from Skoltech, Institute for Problems of Chemical Physics of RAS and MSU, revealing how the modification of the light-absorbing perovskite films affects their optoelectronic properties and photovoltaic performance. It has been shown that replacement of lead in the CH3NH2PbI3 perovskite structure with the univalent cations (e.g. copper or silver) does not affect significantly the solar cell performance. On the contrary, integration of some multivalent cations (e.g. bismuth (III), tin (IV), indium (III)) in the perovskite structure ruins the efficiency of the solar cells down to zero.
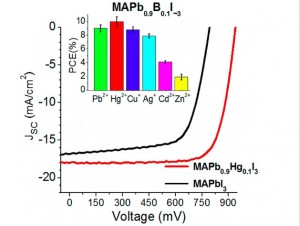
Prof. Pavel Troshin, one of the authors, Skoltech Center for Electrochemical Energy Storage: “Germanium (II) and tin (II) cations incorporated in the active layer of the perovskite solar cells undergo facile disproportionation. These cations are intrinsically unstable in their bivalent state and decompose to metal (0) and M4+ ions. However, the appearance of just trace amounts of the tetravalent metal ions in the photoactive perovskite films results in a dramatic deterioration of their photovoltaic performance. The reason behind that is in the formation of deep traps for mobile charge carriers (electrons) when Pb2+ ions are replaced with the М3+ or М4+ cations in the crystal lattice of the perovskite. As a side product of the performed research, we have shown that a substantial fraction of lead in CH3NH2PbI3 perovskite structure indeed can be replaced by some other bivalent metal ions and this modification even results in substantially improved solar cell performances. Unfortunately, the only model system revealed by now is based on highly toxic mercury. Nevertheless, this result demonstrates a principal feasibility of such kind of replacement, so the research should be continued to explore the full potential of the proposed concept.”
This work was published in Journal of Physical Chemistry Letters.
Contact information:
Skoltech Communications
+7 (495) 280 14 81
