Skoltech researchers have created a promising catalyst to speed up a chemical reaction producing clean hydrogen fuel from the urea contained in wastewater. While that process is known to be catalyzed by various forms of nickel, the Skoltech team has now shown nanowires of that metal embedded in purposely defective carbon nanotubes treated by nitrogen plasma to be particularly fit for withstanding the aggressive alkaline environment of the reaction. This makes the new nickel-based catalyst not just effective but long-lasting, which is an important advantage for future electrolyzers producing hydrogen in a clean way, while also removing urea from wastewater. The findings are reported in the journal Small. The study was supported by the Russian Science Foundation.
“As humanity phases down fossil fuels, one of the main available ways of storing clean energy is in the form of hydrogen fuel. There are multiple industrially relevant chemical reactions used to produce hydrogen, all of them relying on catalysts. Urea oxidation is a promising approach, because it consumes less energy than other competing processes and because it doubles as a wastewater purification technique. We have optimized the catalyst material, reducing its degradation in the harsh reaction environment. This could later be used in electrolyzers producing hydrogen,” said the first author of the study, Aliya Vildanova, a research intern at the Laboratory of Nanomaterials of Skoltech Photonics and a PhD student in the Institute’s Materials Science and Engineering program.
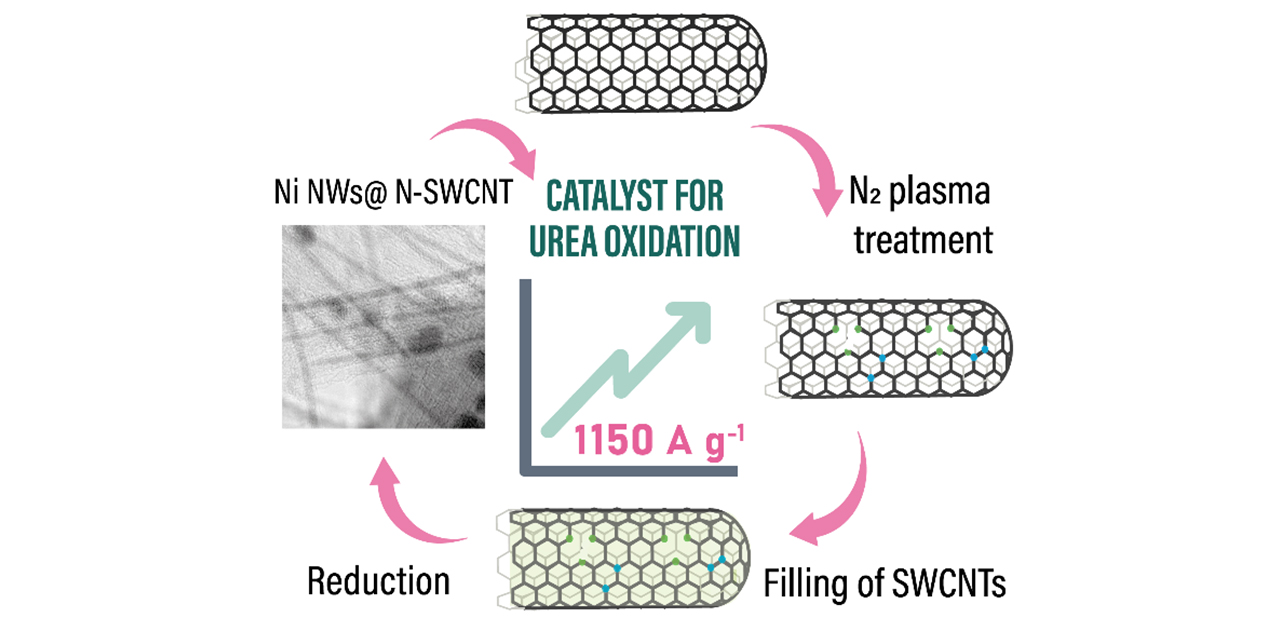
The way the team protected the catalyst from the harsh reaction environment is by embedding nickel into single-walled carbon nanotubes — cylinder-shaped nanosized formations of carbon atoms. This has not been done for that particular metal in that reaction before.
“Noble metals such as platinum and palladium are the most prominent catalysts. Encapsulated inside nanotubes, they have been employed in hydrogen-related catalytic reactions and urea oxidation. While their performance for the latter processes is not outstanding, nickel and nickel oxide demonstrate the best results,” study co-author Assistant Professor Fedor Fedorov from Skoltech Photonics commented. “However, nickel and its oxide are prone to degradation in the harsh environment related to these processes. When nickel is confined inside single-walled carbon nanotubes, we observe much better results — an approach that has not been previously reported as a catalyst for urea oxidation.”
The principal investigator of the study, Professor Albert Nasibulin from Skoltech Photonics said: “We add another twist by exposing the carbon nanotubes to nitrogen plasma for a carefully controlled period of time before embedding nickel. The plasma creates defects in nanotubes, which you can picture as holes in their walls. These defects play two roles: First, they serve as inlets, enabling more nickel to find its way into the nanotubes, resulting in longer nanowires and therefore better performance. Second, the defects turned out to work as active sites of catalysis. That is, by attracting the reactants into close proximity with the nickel catalyst, they further speed up the reaction.”
The optimal mode of plasma treatment that causes all the right defects was first investigated by molecular dynamics simulations and then experimentally confirmed by the researchers.
The team reports that its single-walled carbon nanotubes loaded with nickel exhibit good performance, measured as catalytic activity per unit mass, compared with metal foams and other forms of nickel. The new catalyst is clearly superior in terms of its longevity. When tested, its activity degraded by less than 2% after 1,000 cycles of operation.