Skoltech researchers and their colleagues from the Pasteur Institute and the University of Lorraine, France, have uncovered some of the inner workings of a recently discovered bacterial immune system called PARIS, which can potentially make human pathogens resistant to phage therapy. A promising alternative to antibiotics, it refers to the use of viruses called phages to infect and destroy bacteria that cause disease in humans. Already used to some extent, the approach is expected to make advances when scientists have a better understanding of the interaction between bacteria and phages. That interaction is at the heart of the recent study published in the Philosophical Transactions of the Royal Society B and supported by the Russian Science Foundation.
The overuse and misuse of antimicrobial drugs in humans and farm animals leads to bacteria acquiring resistance to antibiotics. When common pathogens become resistant to previously effective drugs, relatively harmless infections can prove dangerous, particularly for elderly people and other risk groups. And new classes of antibiotics are notoriously difficult to discover.
One possible solution is to do to bacteria what they do to us: infect them. With viruses. This might sound bizarre but specialized viruses called phages, which target specific bacteria, are actually already used to treat some infections. This approach is known as phage therapy.
That said, bacteria are fairly quick to evolve sophisticated immune mechanisms to combat viruses that prey on them. Bacterial immunity has been studied since the 1960s, but so far scientists do not know enough about the interaction between bacteria and viruses to significantly advance phage therapy, for example by purposely “breeding” viruses that would overcome the microbes’ defenses.
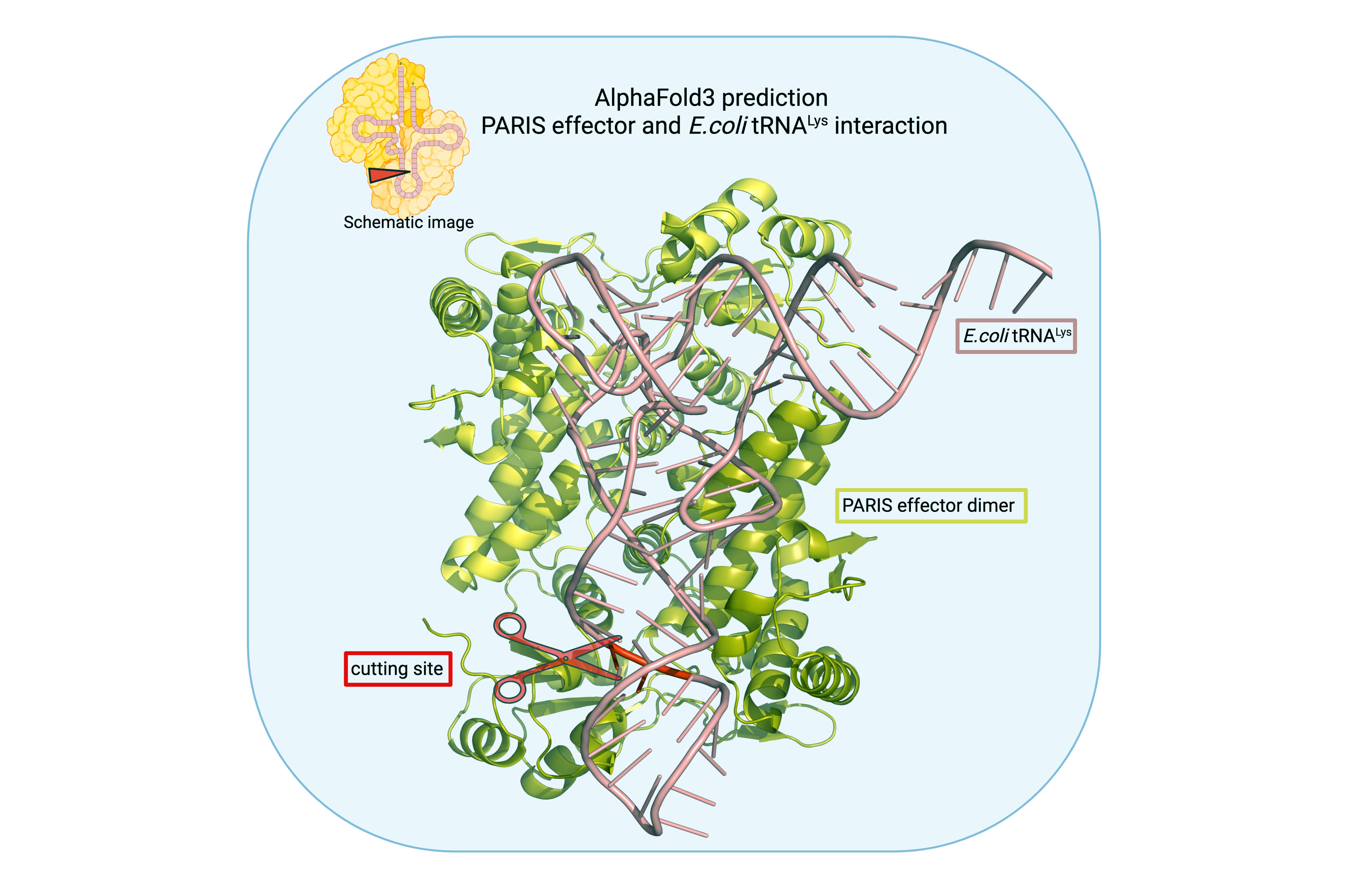
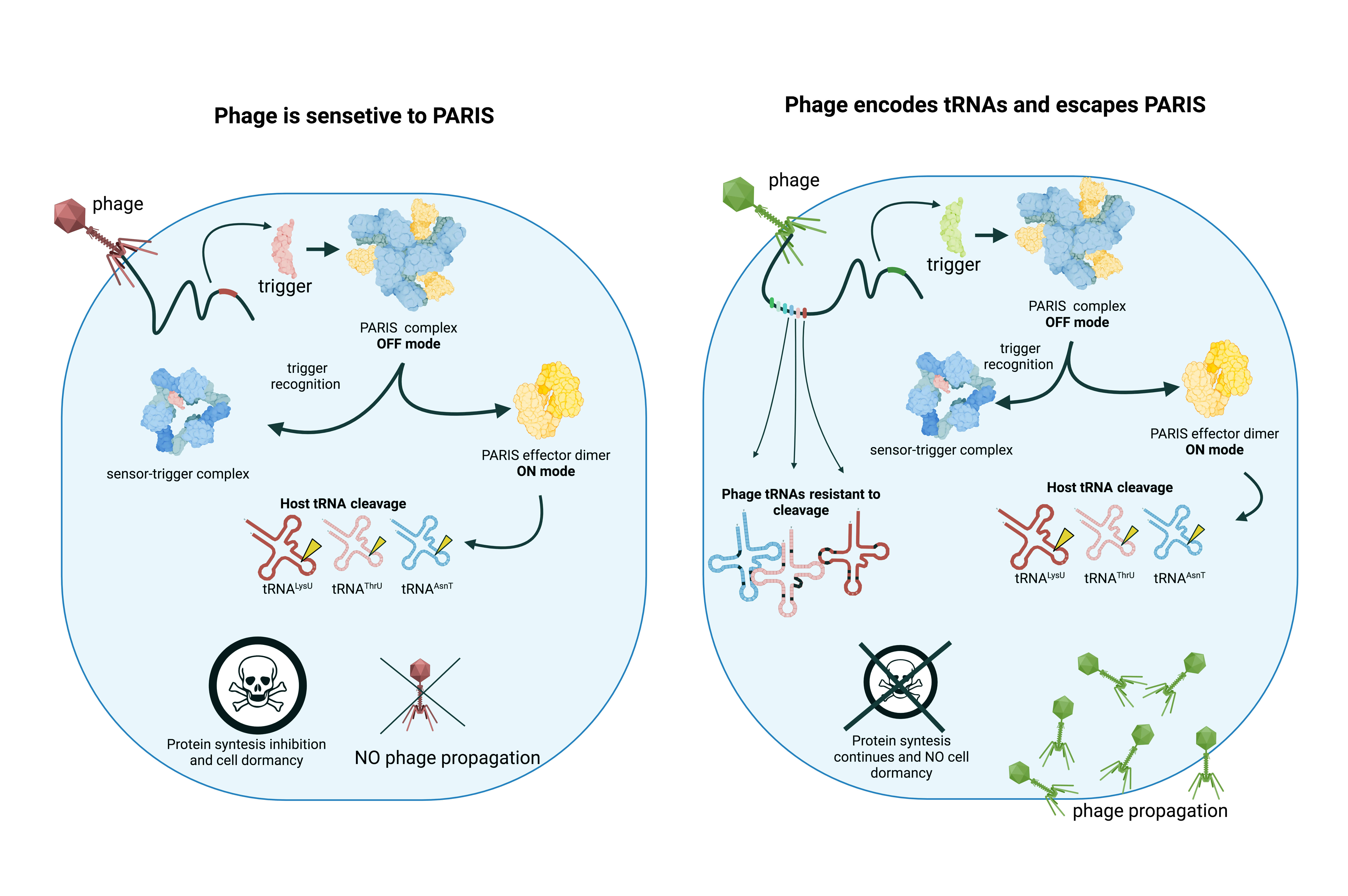
Over the past five to seven years, 150 immune systems of bacteria have been identified. Among them is a system called PARIS, which the team had already studied before. The way it works is by causing the bacterial cell infected by a phage to self-destruct to arrest the spread of the virus. Specifically, the system operates by slashing transfer RNA molecules, which are a key player in protein synthesis. With the protein synthesis impaired, the cell cannot last.
In a devious feat of adaptation, a virus can encode tRNA molecules of its own in its genome and thus bring “replacement tRNA” along into the cell. These substitute molecules are functionally the same, restoring the cell’s ability to assemble proteins. Structurally, they are distinct from bacterial tRNA, rendering them untargetable by the PARIS immune system. Some phage strains do not carry any tRNA. Others encode a substitute molecule for just one bacterial tRNA, and others still can replace as many as 24 tRNAs — almost the entire set typically employed by the E. coli bacterium. Not long ago, scientists were scratching their heads wondering why a phage would ever carry the seemingly useless tRNA at all.