 Using computer modeling, chemists from Skoltech (the Skolkovo Institute of Science and Technology) and MIPT have found out which molecules may be present in the interiors of Uranus, Neptune, and the icy satellites of the giant planets. The scientists discovered that at high pressures, which are typical for the interiors of such planets, exotic molecular and polymeric compounds are formed. These compounds include carbonic acid and orthocarbonic acid, the latter also known as ‘Hitler’s Acid’. The results of the study have been published in the prestigious journal Scientific Reports.
Using computer modeling, chemists from Skoltech (the Skolkovo Institute of Science and Technology) and MIPT have found out which molecules may be present in the interiors of Uranus, Neptune, and the icy satellites of the giant planets. The scientists discovered that at high pressures, which are typical for the interiors of such planets, exotic molecular and polymeric compounds are formed. These compounds include carbonic acid and orthocarbonic acid, the latter also known as ‘Hitler’s Acid’. The results of the study have been published in the prestigious journal Scientific Reports.
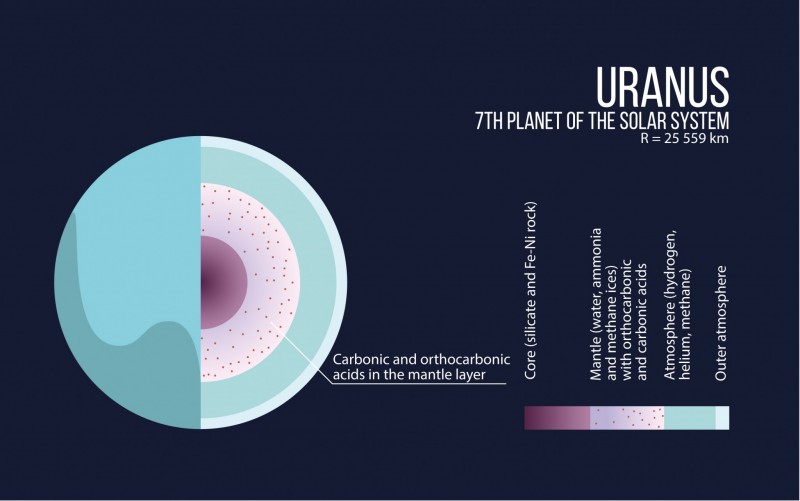
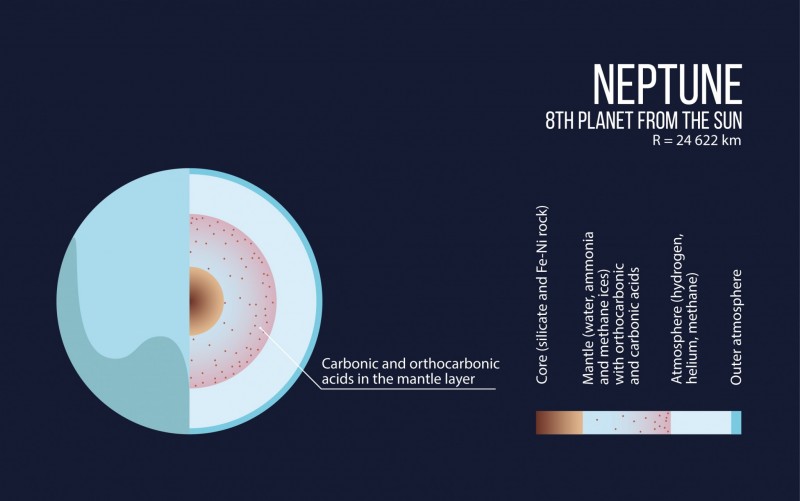
“The smaller gas giants – Uranus and Neptune – consist largely of carbon, hydrogen and oxygen. We have found that at a pressure of several million atmospheres unexpected compounds should form in their interiors. The cores of these planets may largely consist of these exotic materials,” says Artem Oganov, professor of Skoltech the head of MIPT’s Computational Materials Discovery Lab.
A team led by Professor Oganov developed the world’s most universal and powerful algorithm for crystal structure and compound prediction – USPEX (Universal Structure Predictor: Evolutionary Xtallography). In recent years, scientists have used this algorithm to discover several substances that are ‘forbidden’ in classical chemistry and that may be stable at high pressures. These include a number of previously unknown variants of salt – Na3Cl, NaCl3, NaCl7 and even Na3Cl2 andNa4Cl3, as well as exotic new oxides of magnesium, silicon and aluminium which may exist in the interiors of super-Earths.
Now Oganov and Gabriele Saleh (the first author of this study) from MIPT have decided to study the chemical behaviour of the carbon-hydrogen-oxygen system under high pressure. “This is an extremely important system because all organic chemistry ‘rests on’ these three elements, and until now it had not been entirely clear how they behave under extreme pressures and temperatures. In addition, they play an essential role in the chemistry of the giant planets,” says Oganov.
The scientists knew that under atmospheric pressure all compounds of carbon, hydrogen, and oxygen, except for methane, water, and carbon dioxide, are thermodynamically unstable. With an increase in pressure, water and carbon dioxide remain stable, but at pressures above 93 gigapascals (0.93 million atmospheres)methane begins to decompose forming heavy hydrocarbons – ethane, butane, and polyethylene. At a lower pressure – approximately 4 GPa – methane and molecular hydrogen interact, forming co-crystals (where two molecules together create one crystal structure), and at 6 GPa, hydrates – CO-crystals made of methane and water – are formed. To put this into A context, the pressure at the bottom of the Mariana Trench(the deepest part of the world’s oceans) is 108.6 megapascals, which is one thousand times lower.
Saleh and Oganov took on the task of finding all stable compounds in the range up to 400 GPa (around 4 million atmospheres) and discovered several new substances. These included a clathrate (inclusion compound, a type of co-crystal of molecular hydrogen and methane 2CH4:3H2, which is stable in the pressure range 10-215 GPa.
The scientists also found that at a pressure above 0.95 GPa (approximately 10,000 atmospheres), carbonic acid (H2CO3) becomes thermodynamically stable. This is very unusual for a substance that is highly unstable under normal conditions – strong acids are needed for its synthesis and it can only exist in a vacuum at very low temperatures, the authors write.
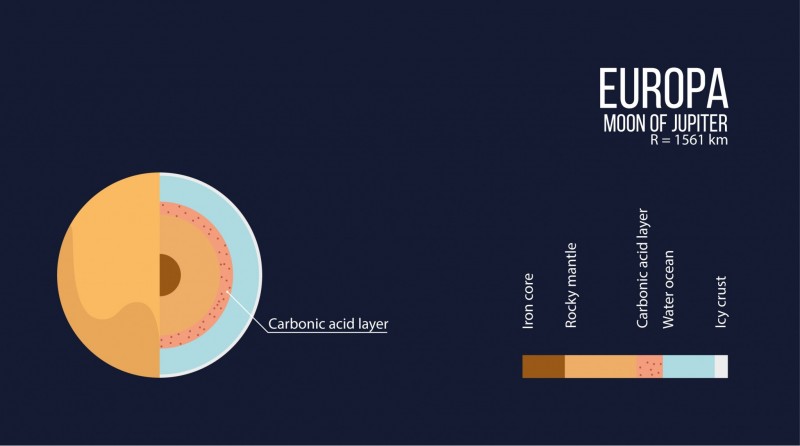
The interiors of the icy satellites of giant planets, such as in Jupiter’s moon Europa, have conditions where carbonic acid could form. On the outside they are covered by a thick layer of ice, and underneath this there is an ocean surrounding a rocky core. According to many scientists, life cannot be ruled out in these oceans.
“It was previously thought that the oceans in these satellites ARE IN direct contact with the rocky core and a chemical reaction took place between them. Our study shows that the core should be ‘wrapped’ in a layer of crystallized carbonic acid, which means that a reaction between the core and the ocean would be impossible,” says Oganov.
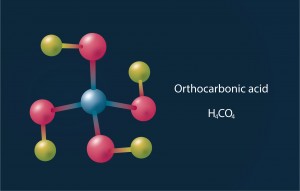
When the pressure rises to 44 GPa, carbonic acid is converted into a polymer that remains stable to at least 400 GPa. In addition, at 314 GPa an exothermic reaction between carbonic acid and water is possible, resulting in orthocarbonic acid (H4CO4). Scientists have not yet been able to produce this compound in laboratories as it is extremely unstable. The molecular structure of orthocarbonic acid resembles a swastika, which is why it is sometimes referred to as ‘Hitler’s Acid’.
“It is possible that the cores of Neptune and Uranus may contain significant amounts of a polymer of carbonic acid and orthocarbonic acid,” says Oganov.
*****
The Skolkovo Institute of Science and Technology (Skoltech) is a private graduate research university in Skolkovo, Russia, a suburb of Moscow. Established in 2011 in collaboration with MIT, Skoltech educates global leaders in innovation, advances scientific knowledge, and fosters new technologies to address critical issues facing Russia and the world. Applying international research and educational models, the university integrates the best Russian scientific traditions with twenty-first century entrepreneurship and innovation.: https://www.skoltech.ru/
Contact information:
Skoltech Communications
+7 (495) 280 14 81