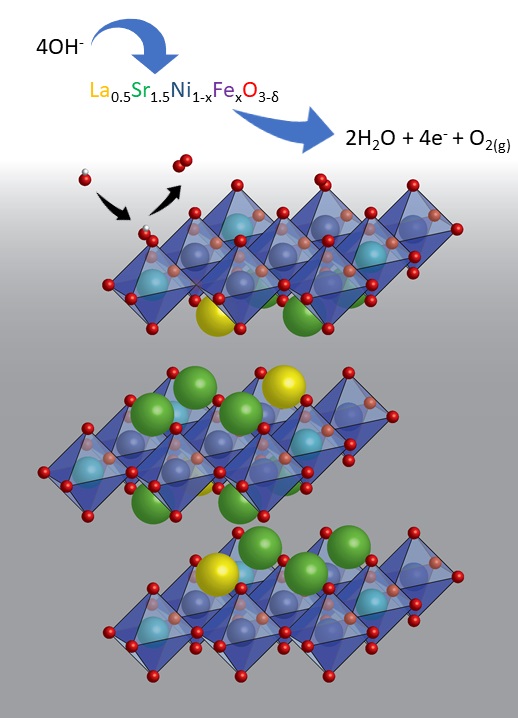
Scheme showing the electrochemical oxidation of hydroxide ions to molecular oxygen. Variation of the amount of Sr, Ni and Fe tunes the catalytic activity for this reaction. Graphic: Robin Forslund // the University of Texas at Austin.
Scientists from the Skolkovo Institute of Science and Technology (Skoltech), the University of Texas at Austin (UTA), and the Massachusetts Institute of Technology (MIT) have reported the discovery of a new metal oxide catalyst that significantly improves the efficiency of water electrolysis in alkaline conditions. The electrolysis of water to evolve oxygen is a reaction that is crucial to enabling emerging renewable energy technologies for the production of hydrogen. Their results were published on 8 August 2018 in the journal Nature Communications (DOI: 10.1038/s41467-018-05600-y).
Currently, there are a number of challenges facing the widespread adoption of water electrolyzers including high energy expenditure, high capital costs and limited durability. For example, the use of expensive precious metals like platinum and iridium limits their large scale implementation.
To overcome the limitations of current materials, the team – led by Skoltech Professor Keith Stevenson – synthesized a series of lanthanum strontium nickel iron Ruddlesden-Popper oxide catalysts (denoted as LSNF) where the properties of the materials could be controllably modified by the substitution of the elements strontium and iron into the nickel based catalyst structure. Through collaborations with researchers at the MIT (Professor Alexei Kolpak), Skoltech (Professor Artem Abakumov) and UTA, the team was able to precisely measure the surface and bulk properties of the catalysts and model how the alkaline water electrolysis reaction advances based on these insights.
Two crucial parameters were identified as important where Sr substitution promotes high catalytic activity by further oxidizing Ni via charge compensation, enhancing Ni-O covalency and electronic conductivity; and the substitution of Fe for Ni introduces the active sites and tunes their electronic properties.
“A lot of work is being done to try and improve the performance of catalysts for oxygen evolution reaction and many types of materials that show greatly improved activities have been reported recently. A big issue, however, is that many of these materials are amorphous and to keep advancing the field and reach a place where devices that use this technology become widespread, we need to take advantage of tools such as computational modeling that can give us deeper insights into the properties that govern how this reaction proceeds, and to do this we need crystalline, well characterized materials. Once we have discovered the fundamental governing principles for the reaction on these well-ordered catalysts we can apply them to many other types of materials as well,” said Robin Forslund, a graduate student at the University of Texas at Austin and a lead author of the study.
Using these parameters, the team developed the catalyst that can perform the water electrolysis reaction approximately one magnitude better than the leading industrial catalyst, IrO2, at a significantly lower cost.
The team found that the enhanced performance of the catalyst was facilitated by the exchange of surface lattice O and that the highly active LSNF materials utilize a lattice-oxygen mediated (LOM) type mechanism. The catalytic effect from the LOM mechanism is further enhanced by the substitution of Fe that causes hybridization of electronic states through Ni-O-Fe bridges. Altogether this demonstrates how the LOM applies to a new series of materials and solidifies the importance of both increased covalency that results in oxygen electron holes, as well as the cross-gap hybridization that leads to enhanced catalytic activity.
While more work needs to be done to further increase the performance of water electrolysis catalysts, this work provides a deeper mechanistic understanding of the materials chemistry of active catalysts. The work also clarifies materials design strategies to accelerate the discovery of additional Earth abundant non-precious metal oxide catalysts. “We now have elucidated important fundamental aspects related to water electrolysis, the catalytic structure and their exceptional electrocatalytic activities of these materials as well as other metal oxide catalysts for alkaline water electrolysis that provides new impetus to overcome the current materials constraints that hinder the pragmatic adoption of water electrolysis, fuel cell and battery technologies,” said Stevenson.
For more information, please contact Skoltech Professor Keith Stevenson at
The Skolkovo Institute of Science and Technology (Skoltech) is a private graduate research university situated on the outskirts of the Russian capital. Established in 2011 in collaboration with MIT, Skoltech cultivates a new generation of researchers and entrepreneurs, promotes advanced scientific knowledge and fosters innovative technology to address critical issues facing Russia and the world. Skoltech applies the best Russian and international research and educational practices,
