Skoltech researchers have developed a novel material for lithium-ion batteries powering electric vehicles. With its ultrahigh energy density, the new cathode material promises longer driving ranges on one charge. Reported in Energy Advances and patented with Rospatent, the material is a familiar nickel-rich layered transition metal oxide, but with an altered microstructure that packs more energy per unit volume.
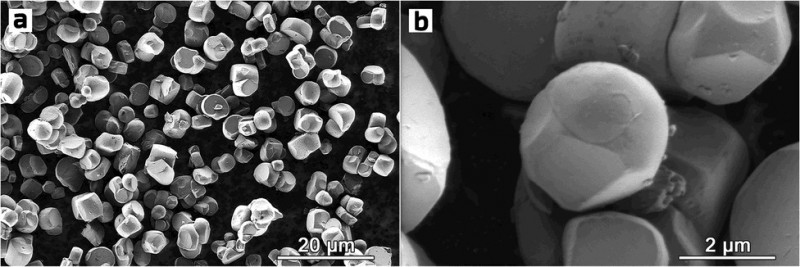
Image. These scanning electron microscopy images at different magnifications reveal the spherelike morphology of the powder particles of NMC622, one of the two materials synthesized in the study by Skoltech researchers. The image is relatively sparsely populated by particles for better visibility, but their spherical morphology means that in an actual battery, they can be compacted to a greater extent than the conventional octahedron-shaped particles, so the material will provide more energy density per unit volume, miniaturizing the battery it is used in. Credit: Ivan Moiseev et al./Energy Advances
“Cathode materials are an important bottleneck as far as electric vehicle batteries are concerned,” principal investigator Professor Artem Abakumov from Skoltech Energy commented. “The cathodes in batteries powering electric cars tend to use layered transition metal oxides, including nickel-rich ones. We improved two commonly used materials of this kind, achieving a 10%-25% increase in energy density. This translates into smaller cathodes, more compact batteries, and therefore greater energy storage capacity for the same volume. As an added bonus, the material is slower to deteriorate.”
The layered oxide originally used in battery cathodes had the formula LiCoO2. In currently used materials, some of the cobalt atoms are replaced by nickel and manganese. These materials are known by technical names such as NMC811, where the numbers reflect the ratio between the three elements in the chemical formula — for example, eight nickel atoms per one atom of manganese and one atom of cobalt. Without changing their chemical composition, Skoltech researchers improved NMC811 and its cousin NMC622 by tweaking the materials’ microstructural organization.
Conventional NMCs are powdered polycrystalline materials, meaning that each secondary particle is made up of randomly oriented grains. The crystal structure within any given grain is nearly flawless, but since no two grains fit perfectly together, some empty spaces inevitably arise at grain boundaries. The single-crystal counterparts of polycrystalline NMCs are just what the name says: Each powder particle is basically one large grain with no wasted spaces in it. These single crystals are usually octahedron-shaped.
“Our material is a single-crystal NMC with spherical particles, combining the best of both worlds as far as maximizing density goes. Unlike polycrystals, the powder particles don’t have internal structure, so there are no wasted spaces at grain boundaries. But on top of that, you can also pack more spherically shaped single crystals into the same limited volume than octahedron-shaped ones, so you get more density on that account, too,” study co-author Skoltech Research Scientist Aleksandra Savina said.
Besides denser packing, the spherical shape of the crystals reduces the area of contact with the electrolyte, minimizing unwanted interactions that over time cause cathode degradation due to crack formation in the particles of conventional NMCs. This should prolong the operating life of the cathode and battery on the whole.
To alter the layered oxide morphology, Skoltech researchers tweaked the synthesis procedure, which relies on the so-called flux method.
Ordinarily, you start with a precursor with homogeneously distributed nickel, manganese, and cobalt. You mix in lithium hydroxide or some other source of lithium, and anneal at high temperature.
“What we do is after adding the lithium-containing compound, we also mix in an inert salt that has a low melting point, and we melt that mixture and anneal at high temperature. We then wash away the salt and anneal again to get rid of the products of any unwanted reactions with water. But the key is that depending on which inert salt is used and in what amount, the particle geometry will change. With conventional synthesis, on the other hand, there’s really not much you can do to affect morphology,” study co-author and Skoltech MSc student Alina Pavlova explained.
The team identified the salt parameters that promoted spherical particle formation. Tests confirmed that the resulting material had the same energy storage capacity per unit mass as the commercial analogues but had greater energy density, enabling more power to be packed into the same limited space.
Asked about their future plans, the researchers said they intended to experiment with particle size, combining smaller and larger spheres for an even denser packing. The team will also pursue layered transition metal oxides that would replace even more cobalt and manganese atoms with nickel, further enhancing energy storage capacity.
The study reported in this story was supported by the Russian Science Foundation.
Contact information:
Skoltech Communications
+7 (495) 280 14 81
